- Kinds of energy
Heat transfer by Conduction
 Consider this illustration. If we place a metal spoon in the hot coffee, soon it begins to feel hot. Why is this so?
Consider this illustration. If we place a metal spoon in the hot coffee, soon it begins to feel hot. Why is this so?
When the spoon (substance) is heated, its particles gain energy and vibrate more vigorously. The particles bump into nearby particles and make them vibrate more. Thermal energy in the vibrating particles or molecules is passed onto nearby particles in a process called conduction.
The flow of energy is from the hot end to the cold end.
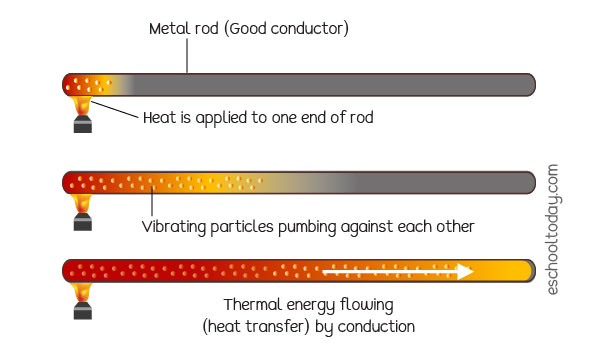
NOTE that the particles in metals are fixed but can vibrate, but their electrons can move about freely. These free electrons transfer the kinetic energy in the particles from the hot areas to the cold areas. Substances that allow thermal energy to move easily through them are called conductors. Metals are good conductors of thermal energy. Substances that do not allow thermal energy to move through them easily are called insulators. Air and plastics are insulators.
Conduction and convection involve the particles in that matter.
