- Kinds of energy
What is Thermal Energy
Matter, is made up of particles or molecules. These molecules move (or vibrate) constantly. A rise in the temperature of matter makes the particles vibrate faster. Thermal energy is what we call the energy that comes from the temperature of matter. The hotter the substance, the more its molecules vibrate, and therefore the higher it’s thermal energy.
For example, a cup of hot tea has thermal energy in the form of kinetic energy from its vibrating particles. When you pour some milk into your hot tea, some of this energy is transferred from the hot tea to the particles in the cold milk. What happens next? The cup of tea is cooler now because it lost thermal energy to the milk. The amount of thermal energy in an object is measured in Joules (J)
We cannot discuss thermal energy without touching on Temperature. Heat and Temperature do not mean the same thing.
Temperature
The temperature of an object is to do with how hot or cold it is, measured in degrees Celsius (°C). Temperature can also be measured in a Fahrenheit scale, named after the German physicist called Daniel Gabriel Fahrenheit (1686 – 1736). It is denoted by the symbol ‘F’. On the Fahrenheit scale, water freezes at 32 °F and boils at 212 °F. On the Celsius scale, water freezes at 0°C and boils at 100°C.
A thermometer is an instrument used to measure the temperature of an object.
Let’s look at this example to see how thermal energy and temperature are related:
A swimming pool at 40°C is at a lower temperature than a cup of tea at 90°C. However, the swimming pool contains a lot more water. Therefore, the pool has more thermal energy than the cup of tea even though the tea is hotter than the water in the pool.
Let us see this example below:
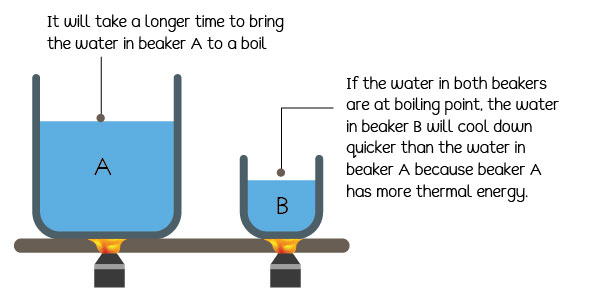
If we want to boil the water in these two beakers, we must increase their temperatures to 100°C. You will notice that it will take longer to boil the water in the large beaker than the water in the small beaker. That is because the large beaker contains more water and needs more heat energy to reach 100°C.
Conduction, Convection, and Radiation
Heat can be transferred from particle to particle or object to object in three different ways. These are Conduction, Convection, and Radiation. Click on the buttons above to learn more.
