- Elements, mixtures, and compounds
- Separating methods
Separation Methods: Simple Distillation
This method is best for separating a liquid from a solution. In a way, the concept is similar to evaporation, but in this case, the vapor is collected by condensation. For example, if you want to separate water from a salt solution, simple distillation would be great for this.
For example: to separate pure water from a salt solution, it works like this:
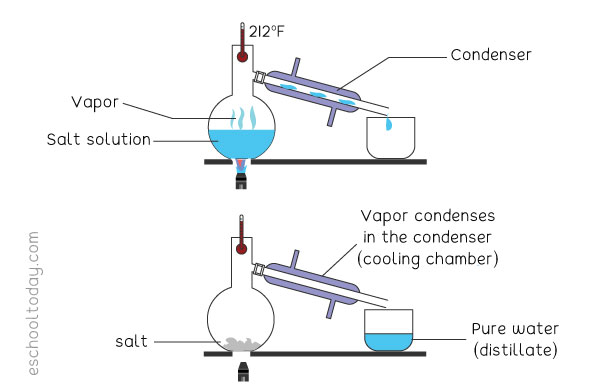
A beaker of the salt solution is heated to the boiling point of the liquid. As it boils, the liquid turns into vapor (gas). The vapor is directed through tubes (condenser) connected to another beaker. As the vapor goes through the tube it is cooled down by running cold water around the tubes. That forces the temperature of the vapor to fall, causing the gas to turn into liquid again (condensation). The liquid is pure at this point, as it is free from salt.
The process continues until all the liquid in the solution turns into vapor, leaving the salt residue. The distilled liquid is called a ‘Distillate’.
