- Elements, mixtures, and compounds
Kinds of mixtures: Solutions (dilute, concentrated, saturated)
A solution is a homogeneous mixture involving a solute and a solvent. The solute is the substance that gets dissolved, and the solvent is the liquid in which the solute dissolves. The solute (may be a liquid or a solid) is broken down completely into individual ions or molecules in a way that can no longer be seen as a separate entity.
A material is said to be soluble if it dissolves completely in a solvent.
For example: If you dissolve salt (solute) in water (solvent), the salt is broken down into Sodium and Chlorine ions within the solvent. This mixture will look and taste the same everywhere in the cup and would have salt and water in the same proportions. In this example, salt is a soluble material.
In the example below, copper sulfate is added to water. What happens when more of the solute is added?
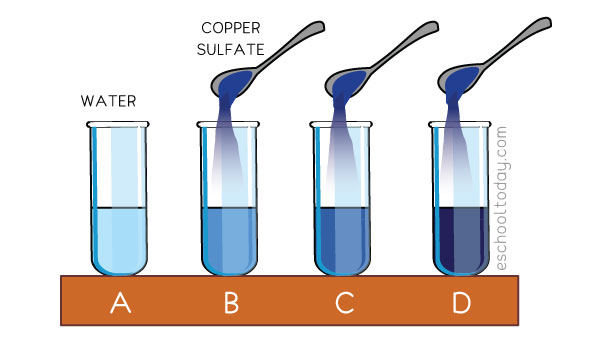
From the illustration above:
A: Water with nothing added. This is not a solution yet.
B: A bit of copper sulfate is added to form a dilute copper sulfate solution.
C: More copper sulfate is added to make it concentrated.
D: More copper sulfate is added to make it saturated.
How can you identify a solution from other mixtures?
1. No particles will be visible.
2. It will have a clear look.
3. Nothing will settle at the bottom of the bottle holding it.
4. It cannot be filtered.
What is a saturated solution?
If you keep adding a solute to a solvent, it gets concentrated. If you keep adding, eventually, no more solute can be dissolved with the temperature remaining constant. Here, the solution is said to be saturated.
