- Elements, mixtures, and compounds
Kinds of mixtures: Alloys
An alloy is a homogeneous mixture of two elements, with one being a metal (solid). For example, Gold as used in jewelry is usually a mixture of gold, silver, and some other metals. When these metals are melted and mixed up, they form alloys. A sample of the new gold alloy will have the same chemical make up like any other sample of that new gold alloy.
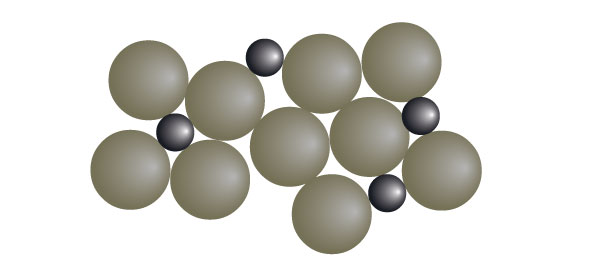
Atomic arrangement in alloys is very irregular and different in sizes, making it harder for its layers to slide over each other.
Stainless steel behaves in a similar way. It is also a mixture of Iron, Chromium, and Nickel. These metals mix up perfectly to become alloys too.
Why are metals mixed into alloys?
The advantage of this mixture is that the alloy tends to have better qualities than the original. For example, an alloy of gold would have better properties in terms of its strength and luster than the unmixed gold.
Common types of alloys.
Amalgam is one common alloy. It is used often by dentists to fill cavities in teeth. The main metal in amalgam is mercury.
Brass is also an alloy, with copper and zinc as its main metals. Brass is usually used for door hinges and electrical plugs.
