- Elements, mixtures, and compounds
- Separating methods
Separation Methods: Fractional Distillation
Similar to simple distillation, fractional distillation is best for separating a solution of two miscible liquids. (Miscible liquids are liquids that dissolve in each other).
The Fractional method takes advantage of the different boiling points of the two liquids.
Example: To separate ethanol from a solution of water and ethanol, it would work like this:
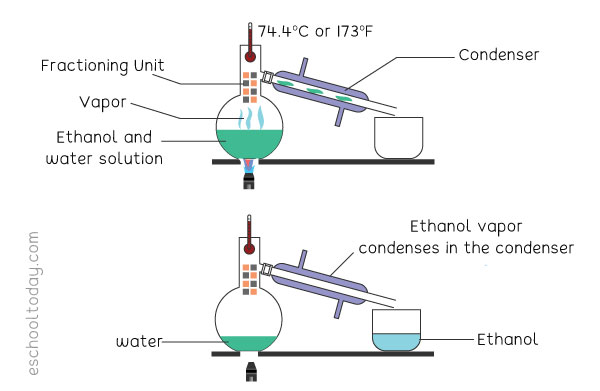
In a mixture of two miscible liquids (like water and ethanol), one liquid will have a lower boiling point (ethanol). In a similar set-up like a simple distillation, the heat is applied to the solution to raise its temperature to the boiling point of the ethanol. That will turn the ethanol in the mixture into a gas. Unlike in the simple distillation set-up, there is a fraction
column between the boiling beaker and the condensing unit. This column is made of layers of glass or beads. The column helps the rising gas to slowly condense and re-evaporate several times before it is collected into the beaker. In the end, the water is separated from the ethanol. Fractional distillation takes a bit more time than simple distillation.
