- Elements, mixtures, and compounds
Kinds of mixtures: Suspensions
A suspension is a heterogeneous mixture of a liquid and a solid. The solid usually do not dissolve and can be very visible to the eye. Sometimes the solids are heavy and large enough for sedimentation (particles settling down in layers) in the container holding it. Unlike colloids, regular agitation is needed to keep the mixture fairly mixed.
An example of a suspension is a mixture of sand and water.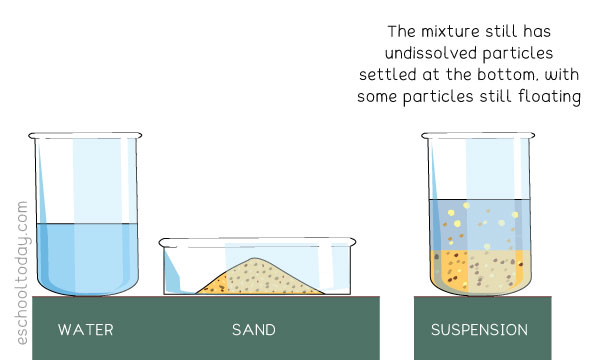
Suspensions often involve 2 phases of matter, because after the solids are mixed with the solvent (liquid) stay the same. Some good characteristics of suspensions are:
1. It is cloudy (not as clear as a solution).
2. It can be filtered.
3. The larger particles settle at the bottom.
4. It is a mixture of two phases.
Note that suspensions can also involve tiny liquid particles in a gas, or tiny solid particle in a gas. Examples include the particulate matter in the atmosphere. Pollutants such as dust particles, soot, salt, or cloud droplets in the atmosphere are all suspensions.
