- Elements, mixtures, and compounds
Kinds of mixtures: Emulsions
An emulsion is a heterogeneous mixture of two or more liquids, in which one ends up as very tiny droplets inside the other. Very often, the liquids involved are not mutually soluble — like adding some water to a bottle of cooking oil. You will notice that, even after some shaking and agitation, it does not dissolve in each other, but appear as bits and pools in the main liquid. Emulsions behave this way.
Emulsions are colloidal systems too.
Here is an illustration of what happens to an oil-water mixture: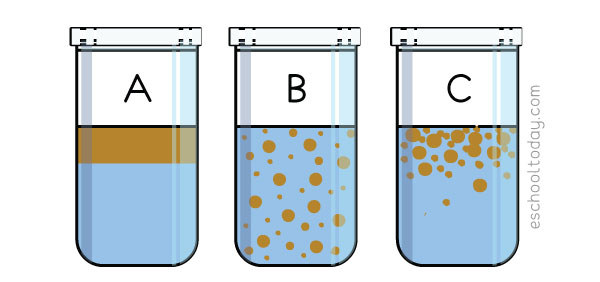
In the illustration above:
A — Liquid with higher density usually stays up. The two liquids are immiscible (They do not mix well)
B — Shake the jar well and the oil bubble will disperse evenly in the water.
C— After a while, the oil particles begin to come together again at the surface.
Emulsions are more viscous than the oil or water that they hold. Examples of emulsions are ice cream, salad dressings, and paints.
“Because a large number of emulsions contain water as one of the two phases, emulsions are classified into two categories: 1) Oil-in-water and 2) Water-in-oil. Oil-in-water emulsions consist of a dispersed phase of oil droplets in a water medium. Water-in-oil emulsions consist of a dispersed phase of oil in a water medium. One can distinguish between emulsion types based on the volume fraction of the two phases.”
Colloids and Emulsions, By Jonathan Collins, Ha Dihn, Heather Gonzales, Ursula Koniges, and Andrew Nordmeyer
- Elements, mixtures, and compounds
Kinds of mixtures: SUSPENSIONS
A suspension is a heterogeneous mixture of a liquid and a solid. The solid usually do not dissolve and can be very visible to the eye. Sometimes the solids are heavy and large enough for sedimentation (particles settling down in layers) in the container holding it. Unlike colloids, regular agitation is needed to keep the mixture fairly mixed.
An example of a suspension is a mixture of sand and water.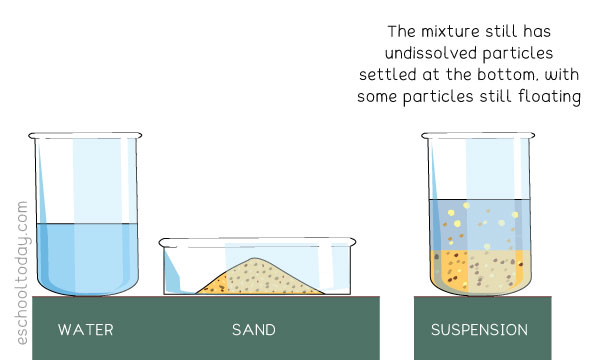
Suspensions often involve 2 phases of matter, because after the solids are mixed with the solvent (liquid) stay the same. Some good characteristics of suspensions are:
1. It is cloudy (not as clear as a solution).
2. It can be filtered.
3. The larger particles settle at the bottom.
4. It is a mixture of two phases.
Note that suspensions can also involve tiny liquid particles in a gas, or tiny solid particle in a gas. Examples include the particulate matter in the atmosphere. Pollutants such as dust particles, soot, salt, or cloud droplets in the atmosphere are all suspensions.
