- Elements, mixtures, and compounds
Elements, compounds, substances, and mixtures
In the study of science, it is essential to understand that all solids are not the same, all liquids are not the same, and all gases are never the same. Additionally, it is vital to understand why all liquids are not the same and why some are purer than others in terms of their chemical makeup or compositions. That is why the classification of Matter is necessary.
Classification of Matter
Matter can be placed into two classes: pure substances and mixtures. This classification is based on the internal composition of that matter. Using composition to describe matter is better than using its state because the internal makeup makes matter unique, and not its phase or state. For example, water can be vapor, solid, or the usual liquid. This means that scientifically, it is not correct to say water is a liquid, even though we all know that water is usually a liquid.
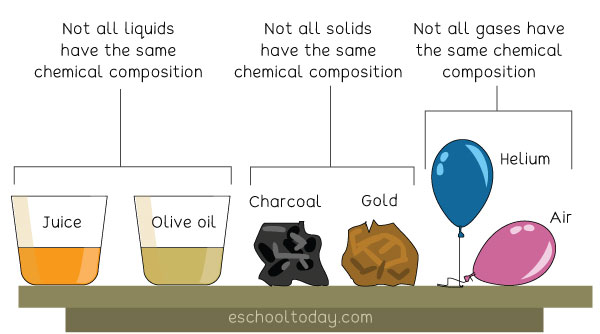
In a similar vein, classifying matter only according to its color, size, or weight is not enough because two identical objects can be of the same color, but their internal makeup may be different. For example, a glass of water from a lake may look and weigh the same as another identical glass of water from another lake — but it does not mean they are the same. They are all water, but the chemical compositions may be very different.
So, in this lesson, we shall see more about the classifications and the various ways and forms in which matter is made up, mixed up, and if they can be separated at all.
First, it is very important to be very clear what some words mean. Let us begin with ‘Elements’
