- Elements, mixtures, and compounds
What is a Compound?
A substance is matter with definite chemical composition and distinct properties. It is matter that is characterized by a constant composition in terms of its molecules, formulae, and atoms, as well as physical properties such as density, refractive index, electric conductivity, melting point, and so on.
A substance can be an element or a compound but NOT a mixture. It can also be matter that exists in its pure form, usually called a pure substance. A few examples of substances include Water (H2O), Hydrogen (H2), and Neon (Ne).
Other examples of chemical substances commonly seen in pure form are salt (sodium chloride), diamond (carbon), and gold.
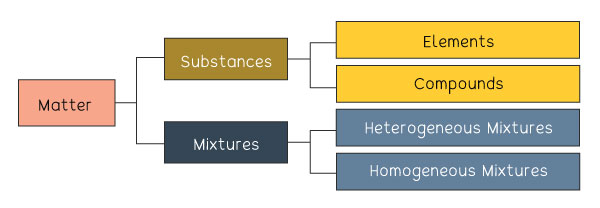
The diagram above shows the classification of matter and where substances fit.
Substances cannot be separated into components by physical separation techniques. Some substances, like water, can be broken down into elements by a chemical reaction (to break chemical bonds). A substance can be a solid, a liquid, a gas, or plasma.
