- The Water Cycle
Hard water or soft water?
Water hardness and softness have nothing to do with its touch and feel. It is about chemical compounds dissolved in it. They are both safe for human consumption.
Pure water (like clean rainwater) is soft water. It only becomes hard water when it comes into contact with rock layers made up of compounds such as calcium or magnesium and dissolves in it.
How does soft water become hard water?
Let us take rainwater as an example. The rainwater may be slightly acidic because of some carbon compounds in the air from pollution. Let’s assume that the rainwater falls on an area with lots of limestone rocks. Limestone contains calcium carbonate (CaCO3). As the water soaks into the limestone layers, calcium compounds dissolve in the acidic rainwater. The water is now hard because it has calcium and magnesium ions in it.
There are various levels of hardness. It all depends on the rock types found in that region.
You will not know if the water is soft or hard by just looking at it. It has to go through a test. In your home, here is how to tell if your water is hard or soft.
Here are a few:
- Hard water does not lather with soap.
- Hard water leaves a lot of scum (some whitish deposits) in the bathtubs and sinks.
- Boiled hard water leaves some limescale residue, which can cause pipes to block over time.
- If the hard water has ferrous irons (oxidized to the ferrous form), it may leave some reddish-brown stains on your clothing after laundry or the bathroom enamel surfaces.
Some people believe that hard water tastes better to drink. It is also known to be good for bones and teeth and can even help reduce heart disease.
Can hard water be softened?
Hard water can be temporal or permanent. Temporal hard water is caused by calcium bicarbonate. This hardness can be removed by just boiling the water, as this converts the carbonate to insoluble carbonate (This can leave a precipitate that can clog your boiler). Permanent hardness is usually caused by other salts.
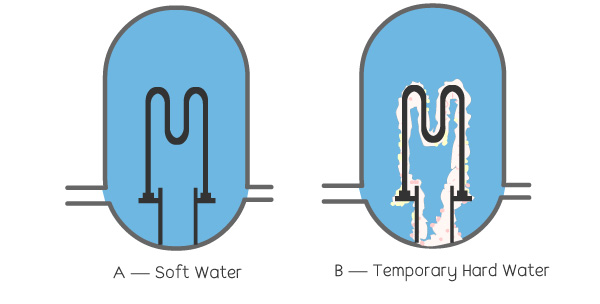
In the illustration above:
A—Soft water does not leave calcium or salt deposits. The boiler, therefore, remains efficient and uses less energy to heat the water.
B—Temporal hard water precipitates calcium deposits, which build up along the coils. The boiler becomes less efficient and consumes more energy.
Permanent hard water can be made soft by adding sodium carbonate (also called washing soda) to it. It will soften both temporal and permanent hardness. In this chemical reaction, the calcium and magnesium ions react with sodium to form a precipitate. That also leaves limescale behind and can clog your pipes. It is not the best idea for softening water in your home.
In another process, ion exchange columns are used. In this chemical reaction, the sodium ions swap places with the calcium ions. The result is water with sodium ions instead of calcium ions. There is no limescale precipitate or residue.
