- Rocks
Chemical Weathering
Chemical weathering is when it involves the reaction of some chemicals on rocks. Some rocks (such as limestone and chalk) are more prone to chemical weathering than others, such as granite. That is because limestone contains minerals such as calcium carbonate, which readily reacts with rainwater. This chemical reaction produces new soluble substances that are easily washed away.
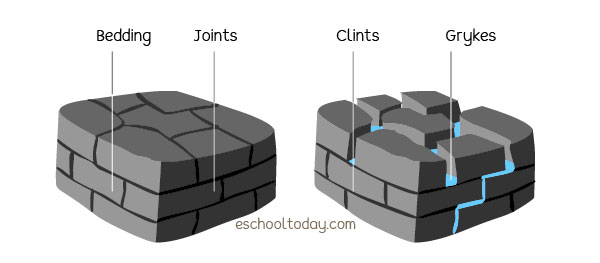
In the example below, see how the beddings of limestone beddings are exposed, because the joints are worn away by carbonic rain, forming clints and grykes, which are common limestone features.
Rainwater contains an acid called carbonic acid. Rain gets acidic because carbon dioxide in the atmosphere dissolves in it. When acidic rainwater falls and stays on rocks, some minerals in the rocks may react chemically with it and cause the rock to weather.
Air pollution that results in more carbon dioxide and sulfur dioxide causes rainwater to become even more acidic. When moisture in the atmosphere dissolves these gases, they form acid rain. When acid rains fall on rocks, the effects are even more than regular rainwater.
Chemical weathering is a key fact in the creation of caves and caverns. Carbonation has also resulted in sinkholes, karst topography, stalactites, and stalagmites.
Hydrolysis is another important reaction associated with chemical weathering. When water (H2O) separates into H+ and OH- ions, the elements can react with ions in the minerals and destroy their atomic compositions, usually forming new minerals. This is what happens when feldspar and hornblende come into contact with water. They form clay, a new mineral.
Chemical weathering can occur even under the top layer of the ground. Acid rainwater can leach into deeper layers underground and come into contact with rocks for chemical reactions to occur.
