- Matter
What are gases?
Gas is everywhere, and it surrounds us. The air around us is a kind of gas. The atmosphere surrounding the earth is gas too. Helium, Oxygen, Carbon dioxide, and water vapor are all gases.
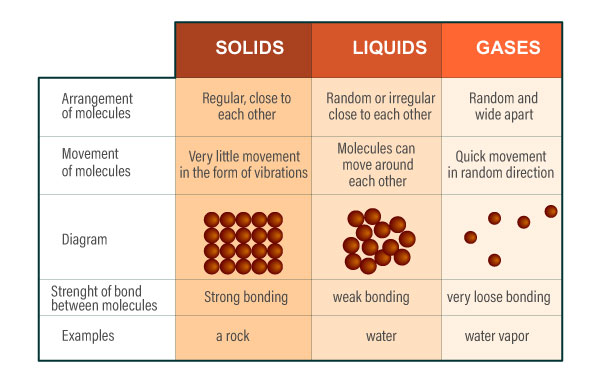
The particles in gases are very different from the particles in solids and liquids.
In gases, the particles are far apart from each other and arranged randomly. The particles also move quickly in all directions. Gases can fill up any container of any shape and size. Gases can be compressed or squashed because the molecules are far from each other. When gas is compressed, the gas molecules move from an area of high pressure to low pressure.
Vapour is also a gas. Gases that are liquid at room temperature, like water, can be classified as vapor. This means they are usually liquid but can vaporize (turn into a gas) under certain conditions.