- Matter
What is a liquid?
The particles in liquids are not as closely bonded, arranged, and fixed in place as solids. The particles in a liquid can flow freely and can mix with particles from other liquids. Liquids have their atoms close together, so they are not very easy to compress.
NOTES
Compressing something means forcing the atoms in it closer together. When you compress an object, you force the atoms closer together.
Liquids, unlike gases, are pulled by gravity to the bottom of the container holding it. They take the shape of the container holding it. You can pour a volume of liquid from one container to the other — it can change shape but the volume will be the same.
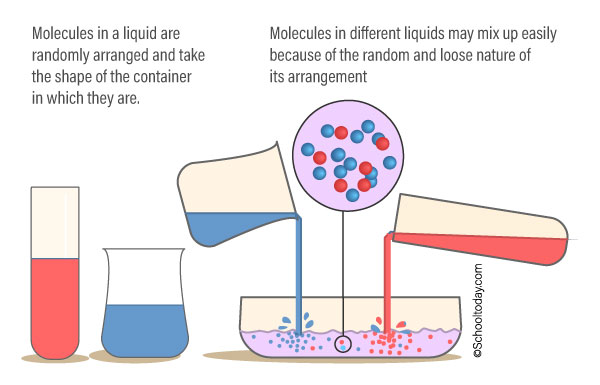
Characteristic of particles in liquids.
The particles are:
- close together, but not as packed as in solids. (They cannot be compressed or squashed)
- arranged randomly.
- move around each other (They flow and take the shape of their container because their particles can move over each other)
- the bonds in a liquid are strong enough to keep the particles close together, but weak enough to let them move around each other.
