- Matter
What are solids?
Solids are simply hard substances, and they are so because of how their molecules are packed together. Examples of solids include rocks, chalk, sugar, pieces of wood, plastic, steel, or a nail. They are all solids at room temperature. They can come in all sizes, shapes, and forms.
Think of ice cubes as an example. They are solids when frozen. They change into a liquid at room temperature.
What about ashes? Is that solid too? Yes, ashes are tiny solid particles that fall off when something burns— like wood ash in the fireplace.
In the diagram below, see how similar the arrangement of molecules is in the piece of rock and wood ash.
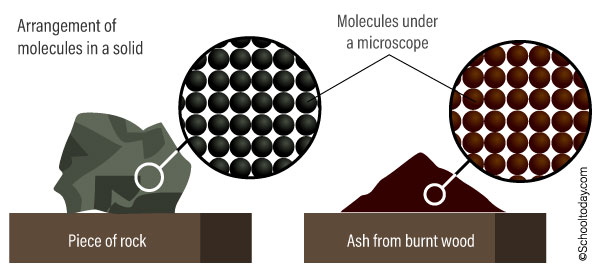
Characteristic of particles (molecules) in a solid.
- The particles are close together (that’s why they cannot be squashed or compressed.
- The particles cannot move freely from place to place (this is why they have a fixed shape).
- The particles are arranged in a regular, distinct pattern.
- The particles are held together by strong forces called bonds (This is why solids do not flow like water. Their particles are only able to vibrate in their position and cannot move from place to place.)
- The particles can vibrate in a fixed position.
Different solids behave slightly differently because they have different properties such as ‘STRENGTH’. This makes solids useful for different things. Look at a pencil eraser—it is solid, but can slightly change shape because the strength of its bonds is slightly weaker than that in a piece of diamond.
