- Matter
Introduction to matter.

Matter is anything, such as a solid, liquid, or gas, that has weight (mass) and occupies space. For anything to occupy space, it must-have volume. Thinking about it, everything on earth has weight and takes up space, and that means everything on earth is matter.
Take a look at this boy blowing bubbles. He is holding a cup with some bubble solution in it. He blows air into the bubble wand, trapping air (gas) in a thin film of the solution.
The boy’s cup, liquid, and the air he is blowing is matter. They all have some weight and volume. Different things of matter behave differently. How and why does the cup, water, or air feel and behave the way they do?
It is because of the individual properties that they are made up of. Their individual properties are held together (bonded) and the strength of the bonds determine why the cup cannot flow (like water) or why air (gas) can be compressed.
But what are their individual properties made of?
Good question. Solids, liquids, and gases are all made up of tiny stuff that the naked eye cannot see, called atoms, molecules, and/or ions. The illustration below is an idea of how atoms, molecules, and ions in matter look like under a microscope.
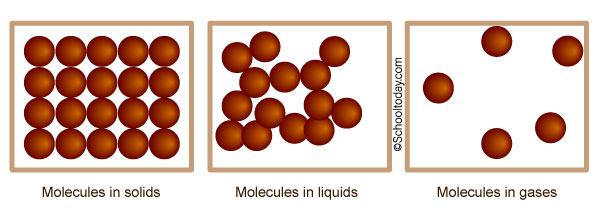
In this lesson, we shall look at the behavior and states of Matter — Solids, Liquids, and Gases, which are known as the three states of matter.
